Startups
From discovery to market, we provide full lifecycle support for your business.
Startup Process Support

Discovery and R&D
Earlier then the first real idea, every start up has to go through R&D.

Proof of Concept
Once you develop your idea, it is now time to trial it.

Non clinical
Work is done to prove your product is safe and effective.
Clinical
Collect Clinical Data in the best correct manner.

Market
Application
Submit a 510(k), PMA, De Novo, HDE, BLA.

Market
Release
Receive Market Notification

Post Market Surveillance
Continued compliance to post market regulations.
Discover all our services
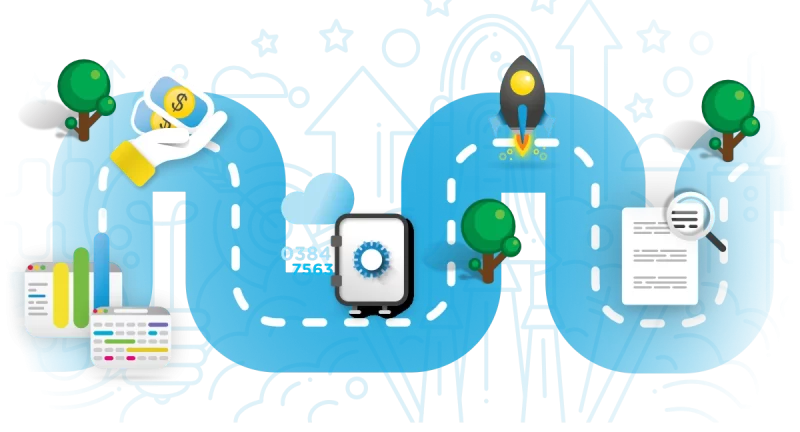
From ideas to market
- Step one: get support in finding grants and funds
- Step two: develop a go-to-market strategy
- Step three: design a Regulatory Strategic Master Plan (RSMP) that fits to your desired market
- Step four: deploy a Quality Assurance and Data Integrity System
- Step five: perform Audits and manage reimbursements