Pharma & API
Meet full compliance with our customized and cost-effective solutions, while ensuring patient safety and business continuity within the entire product life cycle.
Support packages

Data Integrity
Protect records and meet the ALCOA+ requirements.
Digital Governance
All-in-one solutions to coordinate your digital development.

GxP Compliance
Avoid slowdowns and costs by preventively assessing every risk.

Laboratory Excellence
Level up your laboratory to its highest potential.

Engineering & Construction
Design your site in compliance with the latest regulations.

Commissioning & Qualification
A modern and integrated approach to qualification.

Regulatory Affairs
Edit, amend and submit the dossier and registrate worldwide.

Trainings
Perform and customize trainings for your personnel.

Audits
Perform all type of audit, either remotely or onsite.

Clinical Research
Streamline drug development with tailor-made CRO services.

Pharmacovigilance
Comply with regulatory provisions while still being efficient and effective.
Looking for more services?
We are here to help you.
Annex 1 guide
With the latest release of the Second Issue of 2020 GMP Annex I, the standard for all sterile manufacturers has been widely extended and revised.
PQE Group has collected all the informations you need to comply in an exhaustive guide.
Don’t wait: request the download now for free and get all the insights on sterility assurance.


Drug Compounding Compliance
According to the Drug Quality and Security Act of 2013, FDCA establishes all pharmacies need to comply with USP and other regulations, while outsourcing facilities are required to mantain full cGMP Compliance.
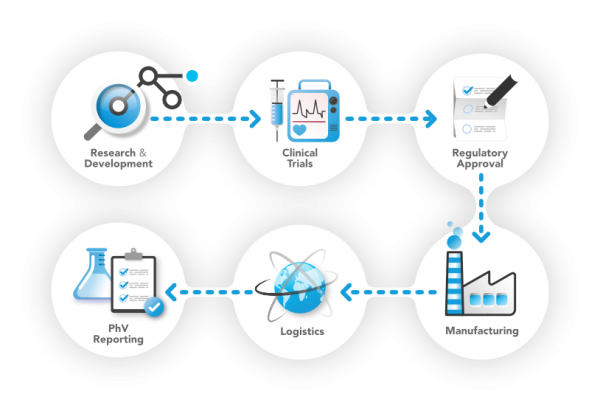
We care about every phase of the product’s lifecycle
In its 20+ years of life, PQE Group has worked with thousands of clients and always applied the latest technologies and discoveries to help companies achieving their full potential.
As an innovation-based agency, we are constantly developing new services and updating our areas of expertise.